
Standardize intake for trade‑ins, buybacks, lease returns, and liquidation with serialized, compliant item records.
Capture condition, testing results, accessories, and documentation consistently at the device level.
Turn structured intake data into clear, trustworthy product listings that meet medical buyers’ requirements.
Sync inventory across warehouses, showrooms, your online store, and marketplaces with real‑time availability and orders.


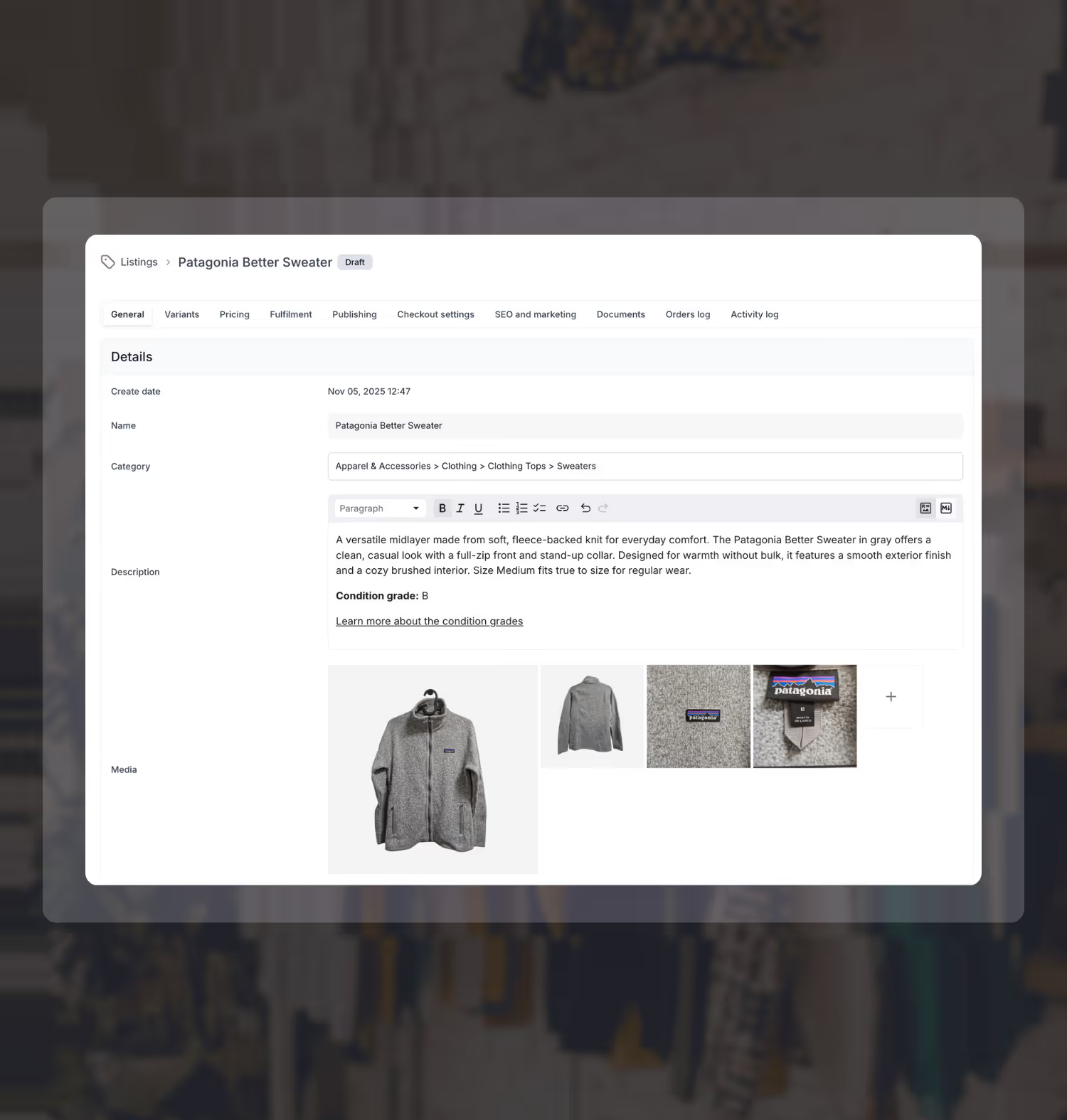

Replace spreadsheets and scattered tools with one easy-to-use system that helps you organize used medical equipment inventory and cross-list items quickly across the resale channels that matter.
Streamline intake, pricing, and listing work while expanding from a physical store to online channels, and stand out by offering a more transparent and reliable buying experience.
Move medical equipment from rental fleet to resale while keeping condition, service history, and compliance fully documented.
Launch and own a certified medical equipment resale or trade-in program that extends asset lifecycles, strengthens customer relationships, and creates new revenue from pre-owned inventory.
TWICE is built around single‑item tracking. Each device receives its own serialized record that stores make, model, serial number, lot or asset IDs, acquisition source, condition, testing data, documentation, and sales history. You always know where a unit came from, where it is, and where it went — without relying on disconnected spreadsheets or manual logs.
Yes. TWICE supports multiple sourcing models in a single system. You can manage hospital and clinic trade‑ins, OEM take‑back programs, lease returns, consignment, and outright purchases. Each intake flow can have its own rules for valuation, ownership, and payouts, while your team works from one consistent intake and inventory process.
TWICE does not replace your clinical, regulatory, or quality systems, but it does provide the commercial infrastructure you need around them. You can capture and store service records, test reports, decontamination certificates, and other documents at the item level, enforce required fields before listing, and maintain a clear audit trail for every sale. This helps support internal compliance processes for high‑value equipment.
TWICE lets you define repeatable workflows for each product category. During intake and testing, teams follow guided steps to record condition, functional checks, accessories, and required documents. Pricing rules can take into account age, brand, modality, condition, and historical performance, while still allowing specialists to override when needed. The result is faster, more consistent valuations without losing expert judgment.
Yes. Many customers keep their existing website, CRM, and sales tools while using TWICE as their recommerce engine in the background. You can link to TWICE‑powered product pages, embed storefront components, or connect via integrations and APIs depending on your setup. TWICE manages inventory, orders, and lifecycle data; your existing systems remain the front‑end for marketing and customer relationships.